Microfluidic test systems for medical diagnostics
1. February 2015 - 31. January 2020
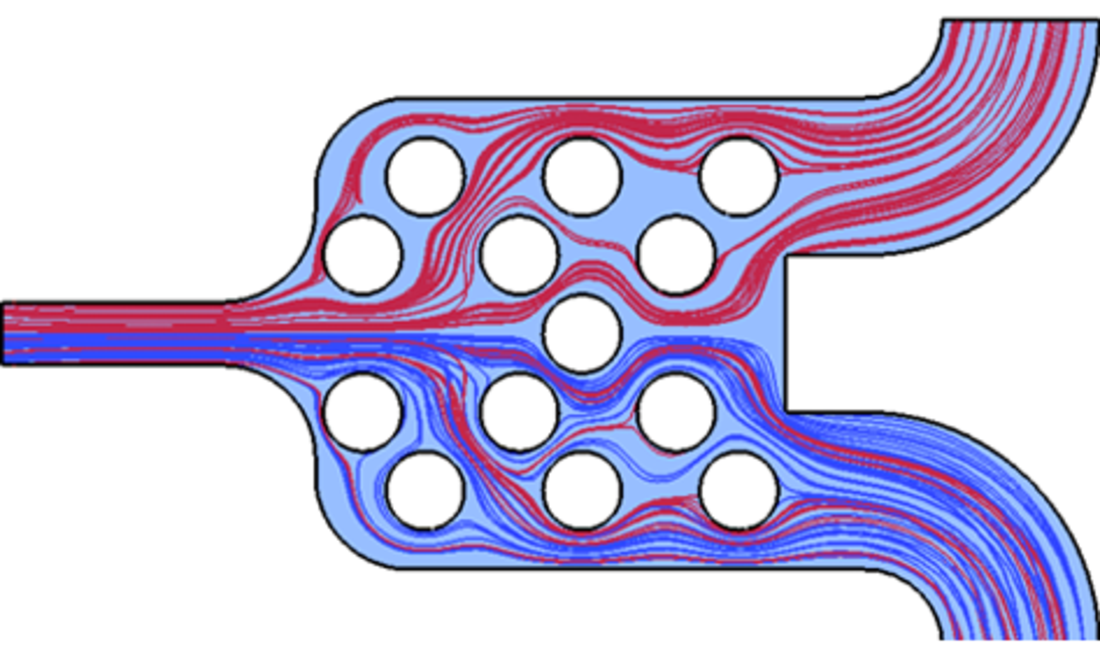
Despite intensive research efforts and high investments, only a few microfluidic testing systems are commercially available and even fewer have been approved as a medical diagnostic system. The study of robust microfluidic methods for the detection and analysis of biological, chemical and physical processes is the focus of this subproject. The aim is to develop standardised microfluidic components that work reliably within different analytical systems, function with consistent quality and are suitable for mass production.
The standardisation and confirmation of suitability for mass production of all microfluidic structures has crucial importance for the success of the InfectoGnostics Research Campus concept. This approach involves creating a simple to use, i.e. easy to implement microfluidic modular system equipped for nearly every test scenario. Only with this modular system consisting of tested geometries optimised for mass-production can the desired point-of-care (POC) test systems for infectious diseases be optimally developed within the research campus.
This research project is part of the campus project "Pneumonia in Immunosuppression"
Project duration
01.02.2015 – 31.01.2020
Project coordination
Ernst Abbe University of Applied Sciences Jena